
|
|
|
|
|
|
|
 |  |

| |
Tensides and Soaps
have a particular molecular structure: They have a water-soluble part (called head) and a liposoluble tail.  Without fat present, these molecules arrange in tiny isolated spheres by connecting their tails. During cleaning, these balls can absorb fat and dirt. |
xirrus simulation - Nanoimaging of soaps
Dishwashing with soap? So antiquated.
A dishwashing detergent shall primarily dissolve fat and dirt. But this is not sufficient for a modern product.
- It also shall tackle persistent crusts.
- At the same time, it shall be skin-friendly.
- It shall cope with the minerals contained in tap water (water hardness).
- It shall drain well without leaving any grey film.
Therefore, it is crucial to meticulously tune active ingredients with each other, concerning not only their effects, but also their dosage.
Nanoimaging depicts the molecular mode of action
Soap molecules tend to linger in groups.
With today's computing power, it is possible to explore detergents including their additives and evaluate their properties when used in calcareous tap water.The following images show concentrated detergent as available on shelf when dissolved in tap water. It is clearly visible how the grey-green tails of the soap molecules group in a sheaves-like structure.
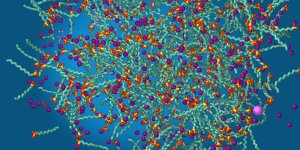 Image 1: Starting point: The components are irregularly distributed.
Tensides: greygreen chains with yellow and red head. Purple balls: sodium ions. Pink balls: calcium ions.
Stray sequestering agents visible as spider-like structures in grey, blue and red. Water not shown.
Image 1: Starting point: The components are irregularly distributed.
Tensides: greygreen chains with yellow and red head. Purple balls: sodium ions. Pink balls: calcium ions.
Stray sequestering agents visible as spider-like structures in grey, blue and red. Water not shown.
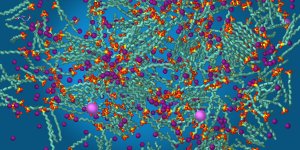 Image 2: The same components after a short time of a few nanoseconds.
Self-assembly takes place. Several sheaves-like bunches of soap tails have already formed.
The heads and ions in reddish colors concentrate at some spots.
Image 2: The same components after a short time of a few nanoseconds.
Self-assembly takes place. Several sheaves-like bunches of soap tails have already formed.
The heads and ions in reddish colors concentrate at some spots.
The restructuring of a soap solution after adding water is so quick such that the interplay between active ingredients can be estimated. As a result, it can be determined which particular ingredients cooperate well in which concentration. Such a result would be difficult to obtain by experimental measuring equipment.
The computer laboratory for product developers
Are you developing new formulations? Then you are often confronted with contradictory requirements to the ingredients. Which ones impede each others effect? Which ones potentiate synergetically?Our computer laboratory provides answers to such questions, both in picture by nanoimaging the molecular processes, and quantitatively by precise analyses.
Need persuasion? Contact us.
| Feedback |
